Catalysis
Background to the schools Wikipedia
SOS Children has tried to make Wikipedia content more accessible by this schools selection. Sponsoring children helps children in the developing world to learn too.
In chemistry and biology, catalysis is a way of accelerating the rate of a chemical reaction by means of contacting the reactants with a substance called a catalyst, which itself is not consumed by the overall reaction. More generally, one may at times call anything that accelerates a process, a "catalyst" (From the Greek καταλύειν, meaning to annul or to untie or to pick up).
A catalyst provides an alternative route to products, the catalytic route being subject to lower activation energy than in the uncatalyzed reaction. A lowered activation energy increases the reaction rate. Catalysts generally change in the course of a reaction but are regenerated.
A good example of a catalyst usage is in the disproportionation of hydrogen peroxide to give water and oxygen:
- 2 H2O2 → 2 H2O + O2
This reaction is slow, as shown by the fact that one can buy solutions of hydrogen peroxide. Upon the addition of manganese dioxide to hydrogen peroxide, the reaction occurs rapidly as signaled by effervescence of oxygen. In demonstrations, the evolved oxygen is detectable by its effect on a glowing splint. The manganese dioxide may be recovered, and re-used indefinitely, thus it is a catalyst — it is not consumed by the reaction. (The H2O2 sold as a sterilizing agent in drugstores is too dilute for this to work dramatically.)
A promoter chemically modifies a catalyst but is not itself a catalyst. An inhibitor reduces the effectiveness of (or slows down the effect of) a catalyst.
History
The phrase catalysed processes was coined by Jöns Jakob Berzelius in 1836 to describe reactions which are accelerated by substances which remain unchanged after the reaction. Other early chemists involved in catalysis were Alexander Mitscherlich who in 1831 referred to contact processes and Johann Wolfgang Döbereiner who spoke of contact action and whose lighter based on hydrogen and a platinum sponge became a huge commercial success in the 1820’s. Humphrey Davy discovered the use of platinum in catalysis. In the 1880s, Wilhelm Ostwald at Leipzig University started a series of systematic investigations into reactions that were catalyzed by the presence of acids and bases, and found both that chemical reactions occur at finite rates, and that these rates can be used to determine the strengths of acids and bases. For this work, Ostwald was awarded the 1909 Nobel Prize in Chemistry.
Typical mechanism
Catalysts generally react with one or more reactants to form an intermediate that subsequently give the final reaction product, in the process regenerating the catalyst. The following is a typical reaction scheme, where C represents the catalyst, A and B are reactants, and D is the product of the reaction of A and B:
- A + C → AC (1)
- B + AC → ABC (2)
- ABC → CD (3)
- CD → C + D (4)
Although the catalyst (C) is consumed by reaction 1, it is subsequently produced by reaction 4, so for the overall reaction:
- A + B → D
Catalytic cycles
A catalytic cycle or catalytic mechanism is a reaction mechanism which involves a catalyst. Catalytic cycles are central to any discussion of catalysis, be it in biochemistry, organometallic chemistry, or solid state chemistry.
Often, a so-called sacrificial catalyst is also part of the reaction system with the purpose of regenerating the true catalyst in each cycle. As the name implies the sacrificial catalyst is not regenerated and is instead irreversibly consumed. This sacrificial compound is also known as a stoichiometric catalyst when added in stoichiometric quantities compared to the main reactant. Usually the true catalyst is an expensive and complex molecule and added in quantities as small as possible. The stoichiometric catalyst on the other hand should be cheap and abundant.
Catalysts and reaction energetics
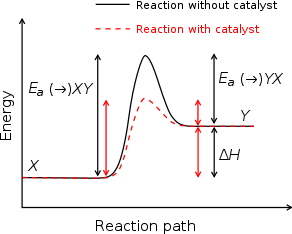
Catalysts work by providing an (alternative) mechanism involving a different transition state and lower activation energy. The effect of this is that more molecular collisions have the energy needed to reach the transition state. Hence, catalysts can perform reactions that, albeit thermodynamically feasible, would not run without the presence of a catalyst, or perform them much faster, more specific, or at lower temperatures. This can be observed on a Boltzmann distribution and energy profile diagram. This means that catalysts reduce the amount of energy needed to start a chemical reaction.
Catalysts cannot make energetically unfavorable reactions possible — they have no effect on the chemical equilibrium of a reaction because the rate of both the forward and the reverse reaction are equally affected (see also thermodynamics). The net free energy change of a reaction is the same whether a catalyst is used or not; the catalyst just makes it easier to activate.
The SI derived unit for measuring the catalytic activity of a catalyst is the katal, which is moles per second. The degree of activity of a catalyst can also be described by the turn over number (or TON) and the catalytic efficiency by the turn over frequency (TOF). The biochemical equivalent is the enzyme unit.
For more information on the efficiency of enzymatic catalysis see the Enzyme#Kinetics section.
Autocatalysis
In autocatalysis, a reaction produces catalysts.
Types of catalysts
Catalysts can be either heterogeneous or homogeneous. Biocatalysts are often seen as a separate group.
Heterogeneous catalysts are present in different phases from the reactants (for example, a solid catalyst in a liquid reaction mixture), whereas homogeneous catalysts are in the same phase (for example, a dissolved catalyst in a liquid reaction mixture).
Heterogeneous catalysts
A simple model for heterogeneous catalysis involves the catalyst providing a surface on which the reactants (or substrates) temporarily become adsorbed. Bonds in the substrate become weakened sufficiently for new bonds to be created. The bonds between the products and the catalyst are weaker, so the products are released. Different possible mechanisms for reactions on surfaces are known, depending on how the adsorption takes place ( Langmuir-Hinshelwood and Eley-Rideal).
For example, in the Haber process to manufacture ammonia, finely divided iron acts as a heterogeneous catalyst. Active sites on the metal allow partial weak bonding to the reactant gases, which are adsorbed onto the metal surface. As a result, the bond within the molecule of a reactant is weakened and the reactant molecules are held in close proximity to each other. In this way the particularly strong triple bond in nitrogen is weakened and the hydrogen and nitrogen molecules are brought closer together than would be the case in the gas phase, so the rate of reaction increases.
Other heterogeneous catalysts include vanadium(V) oxide in the contact process, nickel in the manufacture of margarine, alumina and silica in the cracking of alkanes and platinum, rhodium and palladium in catalytic converters. Mesoporous silicates have found utility in heterogeneous reaction catalysis because their large accessible surface area allows for high catalyst loading.
In car engines, incomplete combustion of the fuel produces carbon monoxide, which is toxic. The electric spark and high temperatures also allow oxygen and nitrogen to react and form nitrogen monoxide and nitrogen dioxide, which are responsible for photochemical smog and acid rain. Catalytic converters reduce such emissions by adsorbing CO and NO onto catalytic surface, where the gases undergo a redox reaction. Carbon dioxide and nitrogen are desorbed from the surface and emitted as relatively harmless gases:
- 2CO + 2NO → 2CO2 + N2
Many catalysts used in refineries and in petrochemical applications are regenerated and reused multiple times to save costs and energy and to reduce environmental impact from recycling or disposal of spent catalysts.
Homogeneous catalysts
Homogeneous catalysts are in the same phase as the reactants.
In homogeneous catalysis the catalyst is a molecule which facilitates the reaction. The catalyst initiates reaction with one or more reactants to form intermediate(s) and in some cases one or more products. Subsequent steps lead to the formation of remaining products and to the regeneration of the catalyst.
Examples of homogeneous catalysts are:
1) The ion H+(aq) which acts as a catalyst in esterification, as well as in the inverse reaction - hydrolysis of esters such as methyl acetate is catalysed by H+
2) Chlorine free radicals in the break down of ozone. These radicals are formed by the action of ultraviolet radiation on chlorofluorocarbons (CFCs). They react with ozone to form oxygen molecules and regenerate the catalyst radicals. This process destroys the thin layer of stratospheric ozone.
- Cl· + O3 → ClO· + O2
- ClO· + O· → Cl· + O2
3) Oxides of nitrogen in the oxidation of sulfur dioxide to sulfur trioxide by dioxygen in the chamber process.
Biocatalysts
In nature enzymes are catalysts in metabolism. In biochemistry catalysis is also observed with abzymes and ribozymes, deoxyribozymes have also been created in the laboratory.
Biocatalysts can be thought of as a mixture of a homogenous and heterogeneous catalyst. This is because the enzyme is in solution itself, but the reaction takes place on the enzyme surface. Several factors affect the activity of enzymes. The most important are:
- Temperature
- pH
- Enzyme concentration
- Substrate concentration
Electrocatalysts
In the context of electrochemistry, specifically in fuel cell engineering, various metal-rich catalysts are used to promote the efficiency of a half reaction that occurs within the fuel cell. One common type of fuel cell electrocatalyst is based upon tiny nanoparticles of platinum which adorn slightly larger carbon particles. When this type of platinum electrocatalyst is in contact with one of the electrodes in a fuel cell, it increases the rate of the redox half reaction in which oxygen gas is reduced to water (or hydroxide or hydrogen peroxide).
Significance
Catalysis is of paramount importance in the chemical industry. The production of most industrially important chemicals involves catalysis. Two notable commercial processes are the Haber process for ammonia synthesis and the Fischer-Tropsch synthesis. Research into catalysis is a major field in applied science, and involves many fields of chemistry, notably in organometallic chemistry, and physics. Catalysis is important in many aspects of environmental science, from the catalytic converter in automobiles to the alleged causes of the ozone hole. Catalytic, rather than stoichiometric reactions are preferred in environmentally friendly green chemistry due to the reduced amount of waste generated.
Notable examples
Estimates are that 90% of all commercially produced chemical products involve catalysts at some stage in the process of their manufacture.
Manganese dioxide is used in the laboratory to prepare oxygen by the decomposition of hydrogen peroxide to oxygen and water.
Well-known applications of synthetic catalysts are:
- Catalytic converters made from platinum and manganese break down some of the more harmful byproducts of automobile exhaust. The catalysts used are micro-engineered to have large surface areas.
- the Haber process for the synthesis of ammonia from nitrogen and hydrogen, where iron is the catalyst.
Examples of catalysts that perform specific transformations on functional groups:
- Transformations of olefinic groups:
- the Ziegler-Natta catalyst used to mass produce polyethylene and polypropylene.
- the Grubbs' catalyst for olefin metathesis.
- the Monsanto process
- the Wacker process
- the Heck reaction.
These given examples show that different catalysts perform other transformations on the same functional groups, where the reaction would not proceed, proceed very slowly, or proceed in an unselective manner without the presence of the catalyst.
The most common catalyst is the proton. Many transition metals and transition metal complexes are used in catalysis as well.
New directions - organocatalysis
While transition metal catalysts are well established, a new trend is toward organocatalysis which use comparatively simple organic molecules as catalysts. While typically, catalyst loading is much higher than transition metal-based catalysts, the catalysts are usually commercially available in bulk, helping to reduce costs drastically. Organocatalysts of the "new generation" are competitive to traditional metal-containing catalysts and are owing to low product inhibition applicable in substoichiometric quantities. The chemical character of organocatalysts offers new and attractive perspectives and advantages to synthetically working chemists.
Catalytic processes
In 2005, Catalytic processes generated about $900 billion in products worldwide. (pdf)
- Acid-base catalysis
- Catalytic converters made from platinum and rhodium break down some of the more harmful byproducts of automobile exhaust.
- Fuel cells
- Fischer-Tropsch synthesis.
- Haber process (synthesis of ammonia from nitrogen and hydrogen, where ordinary iron is used as a catalyst)
- Hydrogenation
- Methanol synthesis
- Nitric acid production
- Petroleum refining and processing
- Alkylation
- Catalytic cracking - breaking long-chain hydrocarbons into smaller pieces
- Naphtha reforming
- Steam reforming of hydrocarbons to produce synthesis gas
- Sulfuric acid production
- Transesterification
- Olefin polymerisation