
Lithium hydride
Did you know...
SOS believes education gives a better chance in life to children in the developing world too. With SOS Children you can choose to sponsor children in over a hundred countries
| Lithium hydride | |
|---|---|
 |
|
| Identifiers | |
| CAS number | 7580-67-8 |
| Properties | |
| Molecular formula | LiH |
| Molar mass | 7.95 g mol−1 |
| Appearance | colorless to gray solid |
| Density | 0.82 g cm−1, solid |
| Melting point |
692 °C |
| Solubility in water | Reacts |
| Hazards | |
| EU classification | Flammable (F) |
| Related compounds | |
| Other cations | sodium hydride, potassium hydride |
| Related compounds | lithium borohydride, lithium aluminium hydride |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
| Infobox references | |
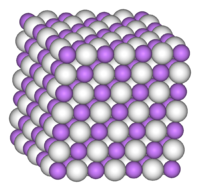
Lithium hydride (Li H) is the compound of lithium and hydrogen. It is a colourless crystalline solid, although commercial samples appear gray. Characteristic of a salt-like hydride, it has a high melting point (689 °C or 1272 ° F). Its density is 780 kilograms per cubic metre. It has a standard heat capacity of 29.73 J/mol*k with thermal conductivity that varies with composition and pressure (from at least 10 to 5 W/m*K at 400 K) and decreases with temperature.
It is a flammable solid and very reactive with water, producing the corrosive compound lithium hydroxide as well as hydrogen.
- LiH + H2O → LiOH + H2
Synthesis
It is produced by reacting lithium metal with hydrogen gas:
- 2 Li + H2
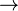 2 LiH
2 LiH
Uses
LiH has numerous uses, as a desiccant, as a precursor for the synthesis of lithium aluminium hydride, in hydrogen generators, as both a coolant and shielding in nuclear reactors, and in the manufacture of ceramics. LiH has the highest hydrogen content (in mass percentage) of any saline hydride. The hydrogen content of LiH is three times that of NaH (though its stoichiometry is identical), because lithium is lighter than sodium, making LiH of interest for hydrogen storage.
The corresponding lithium deuteride, formula LiD, is the fusion fuel in thermonuclear weapons. In warheads of the Teller-Ulam design, LiD is compressed and heated by the explosion of the fission primary to the point where fusion occurs. Lithium deuteride, unlike tritium, is non-radioactive.
Safety
LiH is flammable in air, and it reacts explosively with water to give corrosive LiOH together with hydrogen gas.
In popular culture
In Larry Niven's science fiction book Protector, his character Brennan describes the by-products of a bussard ramjet as being an assortment of strange chemicals including "Lithium Hydride... a normally impossible chemical..." The book was published in 1973.

