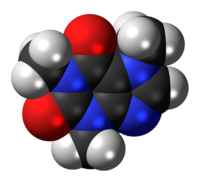
Caffeine
Background Information
SOS Children offer a complete download of this selection for schools for use on schools intranets. SOS Children works in 45 African countries; can you help a child in Africa?
 |
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 |
||||||||||||||||||||||||||
| Systematic (IUPAC) name | ||||||||||||||||||||||||||
| 1,3,7-Trimethyl-1H-purine-2,6(3H,7H)-dione 3,7-Dihydro-1,3,7-trimethyl-1H-purine-2,6-dione |
||||||||||||||||||||||||||
| Clinical data | ||||||||||||||||||||||||||
| AHFS/ Drugs.com | monograph | |||||||||||||||||||||||||
| Pregnancy cat. | C(US) | |||||||||||||||||||||||||
| Legal status | Unscheduled (AU) GSL (UK) OTC (US) | |||||||||||||||||||||||||
| Dependence liability | High | |||||||||||||||||||||||||
| Routes | Oral, insufflation, enema | |||||||||||||||||||||||||
| Pharmacokinetic data | ||||||||||||||||||||||||||
| Bioavailability | 99% | |||||||||||||||||||||||||
| Protein binding | 17% to 36% | |||||||||||||||||||||||||
| Metabolism | demethylation by CYP1A2 | |||||||||||||||||||||||||
| Half-life | 5 hours | |||||||||||||||||||||||||
| Excretion | urine (100%) | |||||||||||||||||||||||||
| Identifiers | ||||||||||||||||||||||||||
| CAS number | 58-08-2 | |||||||||||||||||||||||||
| ATC code | N06B C01 | |||||||||||||||||||||||||
| PubChem | CID 2519 | |||||||||||||||||||||||||
| DrugBank | DB00201 | |||||||||||||||||||||||||
| ChemSpider | 2424 |
|||||||||||||||||||||||||
| UNII | 3G6A5W338E |
|||||||||||||||||||||||||
| KEGG | D00528 |
|||||||||||||||||||||||||
| ChEBI | CHEBI:27732 |
|||||||||||||||||||||||||
| ChEMBL | CHEMBL113 |
|||||||||||||||||||||||||
| Chemical data | ||||||||||||||||||||||||||
| Formula | C8H10N4O2 | |||||||||||||||||||||||||
| Mol. mass | 194.19 g/mol | |||||||||||||||||||||||||
| SMILES | eMolecules & PubChem | |||||||||||||||||||||||||
|
InChI
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
Caffeine is a bitter, white crystalline xanthine alkaloid that acts as a stimulant drug. Caffeine is found in varying quantities in the seeds, leaves, and fruit of some plants, where it acts as a natural pesticide that paralyzes and kills certain insects feeding on the plants, as well as enhancing the reward memory of pollinators. It is most commonly consumed by humans in infusions extracted from the seed of the coffee plant and the leaves of the tea bush, as well as from various foods and drinks containing products derived from the kola nut. Other sources include yerba maté, guarana berries, guayusa, and the yaupon holly.
In humans, caffeine acts as a central nervous system stimulant, temporarily warding off drowsiness and restoring alertness. It is the world's most widely consumed psychoactive drug, but unlike many other psychoactive substances, it is legal and unregulated in nearly all parts of the world. Beverages containing caffeine, such as coffee, tea, soft drinks, and energy drinks, enjoy great popularity. In North America, 90% of adults consume caffeine daily.
Part of the reason caffeine is classified by the Food and Drug Administration as GRAS (generally recognized as safe) is that toxic doses (over 10 grams) are much higher than typically used doses (less than 500 milligrams). Ordinary consumption can have low health risks, even when carried on for years – there may be a modest protective effect against some diseases, including Parkinsons Disease, certain types of cancer. Caffeine can have both positive and negative effects on anxiety disorders. Some people experience sleep disruption if they consume caffeine, especially during the evening hours, but others show little disturbance and the effect of caffeine on sleep is highly variable.
Evidence of a risk to pregnancy is equivocal, but some authorities have concluded that prudent advice is for pregnant women to limit consumption to the equivalent of two cups of coffee per day or less. The American Congress of Obstetricians and Gynecologists (ACOG) concluded in 2010 that caffeine consumption is safe up to 200 mg per day in pregnant women. Caffeine has pressor and mild diuretic effects when administered to people who are not used to it, but regular users develop a tolerance to this effect, and studies have generally failed to support the common notion that ordinary consumption contributes significantly to dehydration. With heavy use, tolerance develops rapidly to the autonomic, but not cognitive or arousal effects of caffeine. The degree to which caffeine can produce clinically significant physical and mental dependence remains a subject of controversy in the medical literature.
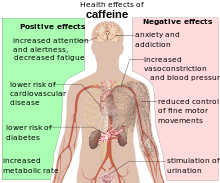
Health effects
Stimulant effects
Caffeine is a central nervous system and metabolic stimulant, and is used both recreationally and medically to reduce physical fatigue and to restore alertness when drowsiness occurs. It produces increased wakefulness, faster and clearer flow of thought, increased focus, and better general body coordination. The amount of caffeine needed to produce effects varies from person to person, depending on body size and degree of tolerance. Effects begin less than an hour after consumption, and a moderate dose usually wears off in about five hours.
Caffeine has a number of effects on sleep, but does not affect all people in the same way. It improves performance during sleep deprivation but may lead to subsequent insomnia. In shift workers it leads to fewer mistakes caused by tiredness. In athletics, moderate doses of caffeine can improve sprint, endurance, and team sports performance, but the improvements are usually not very large. Interestingly, some evidence suggests that coffee does not produce the ergogenic effects observed in other caffeine sources. High doses of caffeine, however, can impair athletic performance by interfering with coordination. Evidence shows that, contrary to common advice, caffeine may be helpful at high altitude.
Physical effects
Consumption of large amounts of caffeine – usually more than 250 mg per day – can lead to a condition known as caffeinism. Caffeinism usually combines caffeine dependency with a wide range of unpleasant physical and mental conditions including nervousness, irritability, restlessness, insomnia, headaches, and heart palpitations after caffeine use.
Coffee consumption is associated with a lower overall risk of cancer. This is primarily due to a decrease in the risks of hepatocellular and endometrial cancer, but it may also have a modest effect on colorectal cancer. There does not appear to be a significant protective effect against other types of cancers, and heavy coffee consumption may increase the risk of bladder cancer. On the other hand, caffeine has been shown to inhibit cellular DNA repair mechanisms., but only at extreme high concentrations (which would be lethal in humans). There is little or no evidence that caffeine consumption increases the risk of cardiovascular disease, and it may somewhat reduce the risk of type 2 diabetes. Drinking four or more cups of coffee per day does not affect the risk of hypertension compared to drinking little or no coffee. However those who drink 1–3 cups per day may be at a slightly increased risk. Caffeine increases intraocular pressure in those with glaucoma but does not appear to affect normal individuals. It may protect people from liver cirrhosis. There is no evidence that coffee stunts a child's growth. Caffeine may increase the effectiveness of some medications including ones used to treat headaches. Similarly, intravenous caffeine is often used in hospitals to provide temporary pain relief for headaches associated caused by low cerebrospinal fluid pressure.
Caffeine consumption during pregnancy does not appear to increase the risk of congenital malformations, miscarriage or growth retardation even when consumed in moderate to high amounts. However as the data supporting this conclusion is of poor quality some suggest limiting caffeine consumption during pregnancy. For example the UK Food Standards Agency has recommended that pregnant women should limit their caffeine intake, out of prudence, to less than 200 mg of caffeine a day – the equivalent of two cups of instant coffee, or one and a half to two cups of fresh coffee. The American Congress of Obstetricians and Gynecologists (ACOG) concluded in 2010 that caffeine consumption is safe up to 200 mg per day in pregnant women. Although the evidence that caffeine may be harmful during pregnancy is equivocal, there is some evidence that the hormonal changes associated with pregnancy slow the metabolic clearance of caffeine from the system, causing a given dose to have longer-lasting effects (as long as 15 hours in the third trimester).
On the positive side, caffeine is the primary treatment of the breathing disorders apnea of prematurity and may also be effective in preventing bronchopulmonary dysplasia in premature infants. The only short-term risk associated with caffeine citrate treatment is a temporary reduction in weight gain during the therapy, and longer term studies (18 to 21 months) have shown lasting benefits of treatment of premature infants with caffeine. While some authors have raised the possibility of subtle long-term problems, follow-up neurological data at 18 months and at five years after neonatal caffeine treatment revealed the opposite; treatment appears to be neuroprotective, as caffeine-treated children were significantly less likely to have cerebral palsy and had reduced rates of language and cognitive delay.
When doses of caffeine equivalent to 2–3 cups of coffee are administered to people who have not consumed caffeine during prior days, they produce a mild increase in urinary output. Because of this diuretic effect, some authorities have recommended that athletes or airline passengers avoid caffeine in order to reduce the risk of dehydration. Most people who consume caffeine, however, ingest it daily. Regular users of caffeine have been shown to develop a strong tolerance to the diuretic effect, and studies have generally failed to support the notion that ordinary consumption of caffeinated beverages contributes significantly to dehydration, even in athletes.
Caffeine has been demonstrated to increase muscle performance: Coso et al. found that a caffeine dose of at least 3 mg/kg in the form of an energy drink improved half-squat and bench-press maximal muscle power.
Psychological effects
The US National Institutes of Health states "too much caffeine can make you restless, anxious, and irritable. It may also keep you from sleeping well and cause headaches, abnormal heart rhythms, or other problems. If you stop using caffeine, you could get withdrawal symptoms. Some people are more sensitive to the effects of caffeine than others. They should limit their use of caffeine. So should pregnant and nursing women."
Four caffeine-induced disorders are recognized by the American Psychiatric Association (APA) including: caffeine intoxication, caffeine-induced sleep disorder, caffeine-induced anxiety disorder and caffeine-related disorder not otherwise specified (NOS). The DSM-IV defines caffeine-induced sleep disorder, as an individual who regularly ingests high doses of caffeine sufficient to induce a significant disturbance in his or her sleep, sufficiently severe to warrant clinical attention. As of 2010 the effect of caffeine on people with ADHD is not known. Some studies have however found a modest protective against Alzheimer disease, but the evidence is inconclusive.
Caffeine can have both negative and positive effects on anxiety disorders. A number of clinical studies have shown a positive association between caffeine and anxiogenic effects and/or panic disorder. At high doses, typically greater than 300 mg, caffeine can both cause and worsen anxiety or, rarely, trigger mania or psychosis. In moderate doses caffeine may reduce symptoms of depression and lower suicide risk. In moderate doses caffeine typically does not affect learning or memory, and can improve cognitive functions, especially in people who are fatigued, possibly due to its effect on alertness. However anxiety sufferers can have high caffeine sensitivity. For some people, anxiety can be very much reduced by discontinuing caffeine use.
Contrary to popular belief, some research suggests that caffeine does not increase motivation in humans, and may even decrease motivation in some.
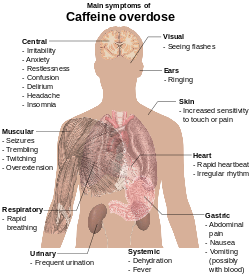
Caffeine toxicity
Caffeine overdose can result in a state of central nervous system over-stimulation called caffeine intoxication ( DSM-IV 305.90),. This syndrome typically occurs only after ingestion of large amounts of caffeine, well over the amounts found in typical caffeinated beverages and caffeine tablets (e.g. more than 400–500 mg per at a time). The symptoms of caffeine intoxication are comparable to the symptoms of overdoses of other stimulants: they may include restlessness, fidgeting, anxiety, excitement, insomnia, flushing of the face, increased urination, gastrointestinal disturbance, muscle twitching, a rambling flow of thought and speech, irritability, irregular or rapid heart beat, and psychomotor agitation. In cases of much larger overdoses, mania, depression, lapses in judgment, disorientation, disinhibition, delusions, hallucinations, or psychosis may occur, and rhabdomyolysis (breakdown of skeletal muscle tissue) can be provoked.
Extreme overdose can result in death. The median lethal dose (LD50) given orally, is 192 milligrams per kilogram in rats. The LD50 of caffeine in humans is dependent on individual sensitivity, but is estimated to be about 150 to 200 milligrams per kilogram of body mass or roughly 80 to 100 cups of coffee for an average adult. Though achieving lethal dose with caffeine would be difficult with regular coffee, there have been reported deaths from overdosing on caffeine pills, with serious symptoms of overdose requiring hospitalization occurring from as little as 2 grams of caffeine. An exception to this would be taking a drug such as fluvoxamine or levofloxacin, which blocks the liver enzyme responsible for the metabolism of caffeine, thus increasing the central effects and blood concentrations of caffeine five-fold. The exact cause of death in such cases is uncertain, but may result from cardiac arrythmia leading to cardiac arrest.
Treatment of severe caffeine intoxication is generally supportive, providing treatment of the immediate symptoms, but if the patient has very high serum levels of caffeine then peritoneal dialysis, hemodialysis, or hemofiltration may be required.
Addiction and tolerance
With repetitive use, physical dependence or addiction may occur. Also, some effects of caffeine, particularly the autonomic effects, decrease over time, a phenomenon known as a tolerance. Tolerance develops quickly to some (but not all) effects of caffeine, especially among heavy coffee and energy drink consumers. Some coffee drinkers develop tolerance to its sleep-disrupting effects, but others apparently do not.
Withdrawal
Withdrawal symptoms – including headache, irritability, inability to concentrate, drowsiness, insomnia, and pain in the stomach, upper body, and joints – may appear within 12 to 24 hours after discontinuation of caffeine intake, peak at roughly 48 hours, and usually last from 2 to 9 days. Withdrawal headaches are experienced by 52% of people who stopped consuming caffeine for two days after an average of 235 mg caffeine per day prior to that. In prolonged caffeine drinkers, symptoms such as increased depression and anxiety, nausea, vomiting, physical pains and intense desire for caffeine containing beverages are also reported. Peer knowledge, support and interaction may aid withdrawal.
In other animals

While safe in humans, caffeine is considerably toxic to various animals, such as dogs and birds.
The increased toxicity of caffeine in some animals is at least partly due to a poorer ability to metabolize the compound.
Caffeine also has a pronounced effect on mollusks, various insects, and spiders.
Chemical properties and biosynthesis
Caffeine is an achiral molecule without stereoisomers.
The two amide groups of caffeine exist predominately as zwitterionic resonance structures where the nitrogen and carbon atoms are double bonded to each other so that both of these nitrogen atoms are essentially planar (in sp2 orbital hybridization). The fused ring system therefore contains a total of ten pi electrons and hence according to Hückel's rule is aromatic.
Caffeine is synthesized in plants from the purine nucleotides AMP, GMP, and IMP. These in turn are transformed into xanthosine and then theobromine, the latter being the penultimate precursor of caffeine.
Being readily available as a byproduct of decaffeination, caffeine is not usually synthesized chemically. If desired, it may be synthesized from dimethylurea and malonic acid.
Pure anhydrous caffeine is a white colorless powder with a melting point of 227–228 °C. Caffeine is moderately soluble in water at room temperature (2 g/100 mL), but very soluble in boiling water (66 g/100 mL). It is also moderately soluble in ethanol (1.5 g/100 mL). It is weakly basic (pKa = ~0.6) requiring strong acid to protonate it.
Pharmacology
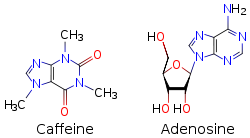
Inside the body caffeine acts through several mechanisms, but its most important effect is to counteract a substance called adenosine that naturally circulates at high levels throughout the body, and especially in the nervous system. In the brain, adenosine plays a generally protective role, part of which is to reduce neural activity levels – for example, there is some evidence that adenosine helps to induce torpor in animals that seasonally hibernate.
Mechanism of action
Adenosine acts as an inhibitor neurotransmitter that suppresses activity in the central nervous system. Consumption of caffeine antagonizes adenosine and increases activity in neurotransmission including acetylcholine, epinephrine, dopamine, serotonin, glutamate, norepinephrine, cortisol, and in higher doses, endorphins which explains the analgesic effect to some users. At very high doses (exceeding 500 milligrams) caffeine inhibits GABA neurotransmission. This evidence explains why caffeine causes anxiety, insomnia, rapid heart and respiration rate.
Because caffeine is both water-soluble and lipid-soluble, it readily crosses the blood–brain barrier that separates the bloodstream from the interior of the brain. Once in the brain, the principal mode of action is as a nonselective antagonist of adenosine receptors (in other words, an agent that reduces the effects of adenosine). The caffeine molecule is structurally similar to adenosine, and is capable of binding to adenosine receptors on the surface of cells without activating them, thereby acting as a competitive inhibitor.
Adenosine is found in every part of the body, because it plays a role in the fundamental adenosine triphosphate (ATP) related energy producing mechanism and is also needed for RNA synthesis, but it has additional functions in the brain. The evidence indicates that brain adenosine acts to protect the brain by suppressing neural activity and by increasing blood flow via receptors located on vascular smooth muscle. Brain adenosine levels are increased by various types of metabolic stress, including lack of oxygen and interruption of blood flow. There is evidence that adenosine functions as a synaptically released neurotransmitter in some parts of the brain; however, stress-related adenosine increases appear to be produced mainly by extracellular metabolism of ATP. Unlike most neurotransmitters, adenosine does not seem to be packaged into vesicles that are released in a voltage-controlled manner, but the possibility of such a mechanism has not been ruled out fully.
Several classes of adenosine receptors have been described, with different anatomical distributions. A1 receptors are widely distributed, and act to inhibit calcium uptake. A2A receptors are heavily concentrated in the basal ganglia, an area that plays a critical role in behaviour control, but can be found in other parts of the brain as well, in lower densities. There is evidence that A 2A receptors interact with the dopamine system, which is involved in reward and arousal. (A2A receptors can also be found on arterial walls and blood cell membranes.)
Beyond its general neuroprotective effects, there are reasons to believe that adenosine may be more specifically involved in control of the sleep-wake cycle. Robert McCarley and his colleagues have argued that accumulation of adenosine may be a primary cause of the sensation of sleepiness that follows prolonged mental activity, and that the effects may be mediated both by inhibition of wake-promoting neurons via A1 receptors, and activation of sleep-promoting neurons via indirect effects on A2A receptors. More recent studies have provided additional evidence for the importance of A2A, but not A1, receptors.
Caffeine, like other xanthines, also acts as a phosphodiesterase inhibitor. A number of potential mechanisms have been proposed for the athletic performance-enhancing effects of caffeine. In the classic, or metabolic theory, caffeine may increase fat utilization and decrease glycogen utilization. Caffeine mobilizes free fatty acids from fat and/or intramuscular triglycerides by increasing circulating epinephrine levels. The increased availability of free fatty acids increases fat oxidation and spares muscle glycogen, thereby enhancing endurance performance. In the nervous system, caffeine may reduce the perception of effort by lowering the neuron activation threshold, making it easier to recruit the muscles for exercise.
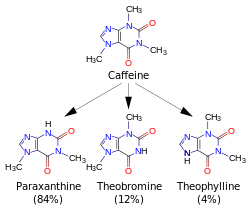
Caffeine metabolites
Metabolites of caffeine also contribute to caffeine's effects. Paraxanthine is responsible for an increase in the lipolysis process, which releases glycerol and fatty acids into the blood to be used as a source of fuel by the muscles. Theobromine is a vasodilator that increases the amount of oxygen and nutrient flow to the brain and muscles. Theophylline acts as a smooth muscle relaxant that chiefly affects bronchioles and acts as a chronotrope and inotrope that increases heart rate and force of contraction.
Metabolism
Caffeine from coffee or other beverages is absorbed by the small intestine within 45 minutes of ingestion and then distributed throughout all tissues of the body. Peak blood concentration is reached within one hour. It is eliminated by first-order kinetics. Caffeine can also be absorbed rectally, evidenced by the formulation of suppositories of ergotamine tartrate and caffeine (for the relief of migraine) and chlorobutanol and caffeine (for the treatment of hyperemesis).
The biological half-life of caffeine – the time required for the body to eliminate one-half of the total amount of caffeine – varies widely among individuals according to such factors as age, liver function, pregnancy, some concurrent medications, and the level of enzymes in the liver needed for caffeine metabolism. It can also be significantly altered by drugs or hormonal states. In healthy adults, caffeine's half-life has been measured with a range of results. Some measures get 4.9 hours, and others are at around 6 hours. Heavy cigarette smokers show a decrease in half-life of 30–50%, oral contraceptives can double it, and pregnancy can raise it even more, to as much as 15 hours during the last trimester. In newborn infants the half-life can be 80 hours or more; however it drops very rapidly with age, possibly to less than the adult value by the age of 6 months. The antidepressant fluvoxamine (Luvox) reduces the clearance of caffeine by more than 90%, and prolongs its elimination half-life more than tenfold; from 4.9 hours to 56 hours.
Caffeine is metabolized in the liver by the cytochrome P450 oxidase enzyme system, in particular, by the CYP1A2 isozyme, into three dimethyl xanthines, each of which has its own effects on the body:
- Paraxanthine (84%): Increases lipolysis, leading to elevated glycerol and free fatty acid levels in the blood plasma.
- Theobromine (12%): Dilates blood vessels and increases urine volume. Theobromine is also the principal alkaloid in the cocoa bean, and therefore chocolate.
- Theophylline (4%): Relaxes smooth muscles of the bronchi, and is used to treat asthma. The therapeutic dose of theophylline, however, is many times greater than the levels attained from caffeine metabolism.
Each of these metabolites is further metabolized and then excreted in the urine. Caffeine can accumulate in individuals with severe liver disease, increasing its half-life.
Some quinolone antibiotics exert an inhibitory effect on CYP1A2, thereby reducing clearance of caffeine and thus increasing blood levels.
A 2011 analysis published by PLoS Genetics reviewed five studies covering more than 47,000 subjects of European descent. Researchers determined that habitual caffeine intake is associated with variations in two genes that regulate how quickly the body processes caffeine. Subjects who had a high-intake mutation of either gene on both chromosomes consumed 40 mg more caffeine per day (equivalent to a can of cola) than people who did not.
Adverse effects
Caffeine has been shown to substantially suppress Calcitriol receptor (Vitamin D Receptor or VDR) protein expression, causing osteoporosis, along with the reduction of all associated hormone response elements. Additionally, VDR reduction has been associated with hair-loss in animal testing.
Detection in biological fluids
Caffeine can be quantified in blood, plasma, or serum to monitor therapy in neonates, confirm a diagnosis of poisoning, or facilitate a medicolegal death investigation. Plasma caffeine levels are usually in the range of 2–10 mg/L in coffee drinkers, 12–36 mg/L in neonates receiving treatment for apnea, and 40–400 mg/L in victims of acute overdosage. Urinary caffeine concentration is frequently measured in competitive sports programs, for which a level in excess of 15 mg/L is usually considered to represent abuse.
Decaffeination

Extraction of caffeine from coffee, to produce decaffeinated coffee and caffeine, is an important industrial process and can be performed using a number of different solvents. Benzene, chloroform, trichloroethylene, and dichloromethane have all been used over the years but for reasons of safety, environmental impact, cost, and flavor, they have been superseded by the following main methods:
- Water extraction: Coffee beans are soaked in water. The water, which contains many other compounds in addition to caffeine and contributes to the flavor of coffee, is then passed through activated charcoal, which removes the caffeine. The water can then be put back with the beans and evaporated dry, leaving decaffeinated coffee with its original flavor. Coffee manufacturers recover the caffeine and resell it for use in soft drinks and over-the-counter caffeine tablets.
- Supercritical carbon dioxide extraction: Supercritical carbon dioxide is an excellent nonpolar solvent for caffeine, and is safer than the organic solvents that are otherwise used. The extraction process is simple: CO2 is forced through the green coffee beans at temperatures above 31.1 °C and pressures above 73 atm. Under these conditions, CO2 is in a " supercritical" state: It has gaslike properties that allow it to penetrate deep into the beans but also liquid-like properties that dissolve 97–99% of the caffeine. The caffeine-laden CO2 is then sprayed with high pressure water to remove the caffeine. The caffeine can then be isolated by charcoal adsorption (as above) or by distillation, recrystallization, or reverse osmosis.
- Extraction by organic solvents: Certain organic solvents such as ethyl acetate present much less health and environmental hazard than chlorinated and aromatic organic solvents used formerly. Another method is to use triglyceride oils obtained from spent coffee grounds.
'Decaffeinated' coffees do in fact contain caffeine, although only about 10 mg per cup as opposed to 85 mg per cup from regular.
History
Caffeine was first isolated from coffee in 1820 by the German chemist Friedlieb Ferdinand Runge, and then independently in 1821 by French chemists Pierre Robiquet, Pierre Pelletier, and Joseph Caventou. Pelletier coined the word "cafeine" from the French word for coffee (café), and this term became the English word "caffeine".
According to Chinese legend, the Chinese emperor Shennong, reputed to have reigned in about 3000 BCE, accidentally discovered tea when he noted that when certain leaves fell into boiling water, a fragrant and restorative drink resulted. Shennong is also mentioned in Lu Yu's Cha Jing, a famous early work on the subject of tea.
The history of coffee has been recorded as far back as the ninth century. During that time, coffee beans were available only in their place of origin, Ethiopia. Legends trace the discovery of coffee either to a Sufi dervish named Omar, or to a goatherder named Kaldi, who observed goats become elated and sleepless at night after grazing on coffee shrubs and, upon trying the berries the goats had been eating, experienced the same vitality. The earliest literary mention of coffee may be a reference to Bunchum in the works of the 9th-century Persian physician al-Razi. The first reliable record of the use of coffee outside Ethiopia comes from Aden, in 1451. The appreciation of coffee as a beverage in Europe dates from the 17th century. The first coffee house in Venice opened some time in the late 1640s. In Britain, the first coffee house was opened in Oxford in 1650. They soon became popular throughout Western Europe, and played a significant role in social relations in the 17th and 18th centuries.
Use of the kola nut, like the coffee berry and tea leaf, appears to have ancient origins. It is chewed in many West African cultures, individually or in a social setting, to restore vitality and ease hunger pangs. In 1911, kola became the focus of one of the earliest documented health scares, when the US government seized 40 barrels and 20 kegs of Coca-Cola syrup in Chattanooga, Tennessee, alleging the caffeine in its drink was "injurious to health". Although the judge ruled in favour of Coca-Cola, two bills were introduced to the U.S. House of Representatives in 1912 to amend the Pure Food and Drug Act, adding caffeine to the list of "habit-forming" and "deleterious" substances, which must be listed on a product's label.
The earliest evidence of cocoa bean use comes from residue found in an ancient Mayan pot dated to 600 BCE. In the New World, chocolate was consumed in a bitter and spicy drink called xocolatl, often seasoned with vanilla, chile pepper, and achiote. Xocolatl was believed to fight fatigue, a belief probably attributable to the theobromine and caffeine content. Chocolate was an important luxury good throughout pre-Columbian Mesoamerica, and cocoa beans were often used as currency.
Xocolatl was introduced to Europe by the Spaniards, and became a popular beverage by 1700. The Spaniards also introduced the cacao tree into the West Indies and the Philippines. It was used in alchemical processes, where it was known as "black bean".
The leaves and stems of the yaupon holly ( Ilex vomitoria) were used by Native Americans to brew a tea called asi or the " black drink". Archaeologists have found evidence of this use stretch back far into antiquity, possibly dating to Late Archaic times.
Discovery
In 1819, the German chemist Friedlieb Ferdinand Runge isolated relatively pure caffeine for the first time; he called it "Kaffebase" (i.e., a base that exists in coffee). In 1821, caffeine was isolated both by French chemist Pierre Jean Robiquet and by another pair of French chemists, Pierre-Joseph Pelletier and Joseph Bienaimé Caventou, according to Swedish chemist Jöns Jacob Berzelius in his yearly journal. Furthermore, Berzelius stated the French chemists had made their discoveries independently of any knowledge of Runge's or each other's work.
Pelletier's article on caffeine was the first to use the term in print (in the French form Caféine). It corroborates Berzelius's account:
Caffeine, noun (feminine). Crystallizable substance discovered in coffee in 1821 by Mr. Robiquet. During the same period – while they were searching for quinine in coffee because coffee is considered by several doctors to be a medicine that reduces fevers and because coffee belongs to the same family as the cinchona [quinine] tree – on their part, Mssrs. Pelletier and Caventou obtained caffeine; but because their research had a different goal and because their research had not been finished, they left priority on this subject to Mr. Robiquet. We do not know why Mr. Robiquet has not published the analysis of coffee which he read to the Pharmacy Society. Its publication would have allowed us to make caffeine better known and give us accurate ideas of coffee's composition ...
German chemist Hermann Emil Fischer (1852–1919) first synthesized caffeine from raw materials in 1895 and two years later, he also derived the structural formula of the compound.
Robiquet was one of the first to isolate and describe the properties of pure caffeine while Pelletier was the first to perform an elemental analysis.
Berzelius later acknowledged Runge's priority in the extraction of caffeine, stating: "However, at this point, it should not remain unmentioned that Runge (in his Phytochemical Discoveries, 1820, pages 146–147) specified the same method and described caffeine under the name Caffeebase a year earlier than Robiquet, to whom the discovery of this substance is usually attributed, having made the first oral announcement about it at a meeting of the Pharmacy Society in Paris. According to Runge, he did this at the behest of Johann Wolfgang von Goethe." In 1827, M. Oudry isolated "theine" from tea, but it was later proved by Mulder and by Carl Jobst that theine was the same as caffeine. The structure of caffeine was elucidated near the end of the 19th century by Hermann Emil Fischer, who was also the first to achieve its total synthesis. This was part of the work for which Fischer was awarded the Nobel Prize in 1902.
Legality
Today, caffeine is legal and available in many forms in all jurisdictions.
Historically, coffee and thus caffeine was illegal for some classes in Mecca in parts of the 16th century, and in the Ottoman empire. Charles II of England tried to ban it in 1676, Frederic II of Prussia banned it in 1777, and coffee was banned in Sweden in the years 1756–1769, 1794–1796, 1799–1802, and 1817–1823. The bans on coffee have often had religious, economic, or political reasons rather than being based on concerns for the well-being of the population.
Religion
Some Seventh-day Adventists, Church of God (Restoration) adherents, and Christian Scientists do not consume caffeine. Some from these religions believe that one is not supposed to consume a non-medical, psychoactive substance, or believe that one is not supposed to consume a substance that is addictive. The Church of Jesus Christ of Latter-day Saints has said the following with regard to caffeinated beverages: "With reference to cola drinks, the Church has never officially taken a position on this matter, but the leaders of the Church have advised, and we do now specifically advise, against the use of any drink containing harmful habit-forming drugs under circumstances that would result in acquiring the habit. Any beverage that contains ingredients harmful to the body should be avoided."
Gaudiya Vaishnavas generally also abstain from caffeine, as it is alleged to cloud the mind and over-stimulate the senses. To be initiated under a guru, one must have had no caffeine, alcohol, nicotine or other drugs, for at least a year.
People who refrain from consuming caffeine, for religious or other reasons, may instead use a substitute that performs a culturally similar role to coffee.
Caffeinated beverages are widely consumed by Muslims today; in the 16th century, some Muslim authorities made unsuccessful attempts to ban them as forbidden "intoxicating beverages" under Islamic dietary laws.