Liquid crystal
About this schools Wikipedia selection
SOS Children have produced a selection of wikipedia articles for schools since 2005. SOS Children has looked after children in Africa for forty years. Can you help their work in Africa?

Liquid crystals are substances that exhibit a phase of matter that has properties between those of a conventional liquid, and those of a solid crystal. For instance, a liquid crystal (LC) may flow like a liquid, but have the molecules in the liquid arranged and/or oriented in a crystal-like way. There are many different types of LC phases, which can be distinguished based on their different optical properties (such as birefringence). When viewed under a microscope using a polarized light source, different liquid crystal phases will appear to have a distinct texture. Each "patch" in the texture corresponds to a domain where the LC molecules are oriented in a different direction. Within a domain, however, the molecules are well ordered. Liquid crystal materials may not always be in an LC phase (just as water is not always in the liquid phase: it may also be found in the solid and gas phase).
Liquid crystals can be divided into thermotropic and lyotropic LCs. Thermotropic LCs exhibit a phase transition into the LC phase as temperature is changed, whereas lyotropic LCs exhibit phase transitions as a function of concentration of the mesogen in a solvent (typically water) as well as temperature .
History
In 1888 Austrian botanical physiologist Friedrich Reinitzer (1858-1927), working at the German University of Prague, was extracting cholesterol from carrots to establish its chemical formula. Reinitzer examined the physico-chemical properties of various derivatives of the cholesterol. Other researchers had observed distinct colour effects on cooling cholesterol derivatives just above the solidification temperature. Reinitzer himself found the same phenomenon in cholesteryl benzoate, but the colors near the solidification of cholesteryl benzoate were not the most peculiar feature. Reinitzer found that cholesteryl benzoate does not melt like other compounds, but had two melting points. At 145.5°C it melted into a cloudy liquid, and at 178.5°C it melted again and the cloudy liquid became clear. The phenomenon was reversible. Seeking help from a physicist, on March 14, 1888, he wrote a letter to Otto Lehmann, at that time a Privatdozent in Aachen. They exchanged letters and samples. Lehmann examined the intermediate cloudy fluid, and reported seeing crystallites. Reinitzer's Viennese colleague von Zepharovich also indicated that the intermediate "fluid" was crystalline. The exchange of letters with Lehmann ended on April 24, with many questions unanswered. Reinitzer presented his results, with credits to Lehmann and von Zepharovich, at a meeting of the Vienna Chemical Society on May 3, 1888 .
By this time Reinitzer had discovered and described three important features of cholesteric liquid crystals (the name coined by Georges Friedel in 1922): the existence of two melting points, the reflection of circularly polarized light, and the ability to rotate the polarization direction of light.
After his accidental discovery, Reinitzer disappeared from the stage. Research was continued by Lehmann, who realised that he had encountered a new phenomenon and was in a position to launch an investigation of it. In his postdoctoral years he had acquired expertise in crystallography. The principal weapon in his scientific arsenal was experimental microscopy in which Lehmann was adept. It was Lehmann's microscope which had attracted Reinitzer's attention. Lehmann started a systematic study, first of cholesteryl benzoate, and then of related compounds which exhibited the double-melting phenomenon. He was able to make observations in polarised light, and his microscope possessed a hot stage enabling high temperature observations. The intermediate cloudy phase clearly sustained flow, but other features, particularly the signature under a microscope, convinced Lehmann that he was dealing with a solid. By the end of August 1889 he had his article ready for submission to the Zeitschrift für Physikalische Chemie .
Liquid crystals were not popular among scientists in the early 20th century and the material remained a pure scientific curiosity for about 80 years.
For more details on the history of liquid crystals see: Tim Sluckin, Ueber die Natur der kristallinischen Flüssigkeiten und flüssigen Kristalle (The early history of liquid crystals), Bunsen-Magazin, 7.Jahrgang, 5/2005 and the History of Liquid Crystals website.
In 1969, Hans Kelker succeeded in synthesizing a substance that has a nematic phase at room temperature, MBBA (p-Methoxybenzyliden-p’-n-butylanilin), the well known "fruit-fly" of liquid crystal research . The next step to commercialization of liquid crystal displays was the synthesis of further chemically stable substances (cyanobiphenyls) with low melting temperatures by George Gray .
In 1991, when liquid crystal displays were already well established in our everyday life, Pierre-Gilles de Gennes (1932-2007) received the Nobel Prize in physics "for discovering that methods developed for studying order phenomena in simple systems can be generalized to more complex forms of matter, in particular to liquid crystals and polymers". An illustrative introduction into liquid crystals, their history and unique physical properties is available on Nobelprize.org.
Liquid crystal phases
The various LC phases (called mesophases) can be characterized by the type of ordering that is present. One can distinguish positional order (whether molecules are arranged in any sort of ordered lattice) and orientational order (whether molecules are mostly pointing in the same direction), and moreover order can be either short-range (only between molecules close to each other) or long-range (extending to larger, sometimes macroscopic, dimensions). Most thermotropic LCs will have an isotropic phase at high temperature. That is, heating will eventually drive them into a conventional liquid phase characterized by random and isotropic molecular ordering (little to no long-range order), and fluid-like flow behaviour. Under other conditions (for instance, lower temperature), an LC might inhabit one or more phases with significant anisotropic orientational structure and short-range orientational order while still having an ability to flow.
The ordering of liquid crystalline phases is extensive on the molecular scale. This order extends up to the entire domain size, which may be on the order of micrometres, but usually does not extend to the macroscopic scale as often occurs in classical crystalline solids. However, some techniques (such as the use of boundaries or an applied electric field) can be used to enforce a single ordered domain in a macroscopic liquid crystal sample. The ordering in a liquid crystal might extend along only one dimension, with the material being essentially disordered in the other two directions.
Thermotropic liquid crystals
Thermotropic phases are those that occur in a certain temperature range. If the temperature is raised too high, thermal motion will destroy the delicate cooperative ordering of the LC phase, pushing the material into a conventional isotropic liquid phase. At too low a temperature, most LC materials will form a conventional (though anisotropic) crystal. Many thermotropic LCs exhibit a variety of phases as temperature is changed. For instance, a particular mesogen may exhibit various smectic and nematic (and finally isotropic) phases as temperature is increased. An example of a compound displaying thermotropic LC behaviour is para-azoxyanisole.
Nematic phase
One of the most common LC phases is the nematic, where the molecules have no positional order, but they have long-range orientational order. Thus, the molecules flow and their centre of mass positions are randomly distributed as in a liquid, but they all point in the same direction (within each domain). Most nematics are uniaxial: they have one axis that is longer and preferred, with the other two being equivalent (can be approximated as cylinders). Some liquid crystals are biaxial nematics, meaning that in addition to orienting their long axis, they also orient along a secondary axis.
The word nematic comes from the Greek νημα, which means 'thread.' This term originates from the thread-like topological defects observed in nematics, which are formally called ' disclinations.' Nematics also exhibit so-called hedgehog topological defects.
Nematics have fluidity similar to that of ordinary (isotropic) liquids but they can be easily aligned by an external magnetic or electric field. An aligned nematic has the optical properties of a uniaxial crystal and this makes them extremely useful in liquid crystal displays (LCD).
Smectic phases
The smectic phases, which are found at lower temperatures than the nematic, form well-defined layers that can slide over one another like soap. The smectics are thus positionally ordered along one direction. In the Smectic A phase, the molecules are oriented along the layer normal, while in the Smectic C phase they are tilted away from the layer normal. These phases are liquid-like within the layers. There is a very large number of different smectic phases, all characterized by different types and degrees of positional and orientational order.
Chiral phases
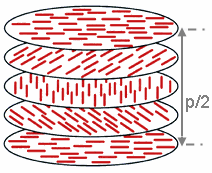
The chiral nematic phase exhibits chirality (handedness). This phase is often called the cholesteric phase because it was first observed for cholesterol derivatives. Only chiral molecules (i.e.: those that lack inversion symmetry) can give rise to such a phase. This phase exhibits a twisting of the molecules perpendicular to the director, with the molecular axis parallel to the director. The finite twist angle between adjacent molecules is due to their asymmetric packing, which results in longer-range chiral order. In the smectic C* phase (an asterisk denotes a chiral phase), the molecules have positional ordering in a layered structure (as in the other smectic phases), with the molecules tilted by a finite angle with respect to the layer normal. The chirality induces a finite azimuthal twist from one layer to the next, producing a spiral twisting of the molecular axis along the layer normal.
The chiral pitch, p, refers to the distance over which the mesogens undergo a full 360° twist (but note that the structure of the chiral nematic phase repeats itself every half-pitch, since in this phase directors at 0° and +/-180° are equivalent). The pitch, p, typically changes when the temperature is altered or when other molecules are added to the LC host (an achiral LC host material will form a chiral phase if doped with a chiral material), allowing the pitch of a given material to be tuned accordingly. In some liquid crystal systems, the pitch is of the same order as the wavelength of visible light. This causes these systems to exhibit unique optical properties, such as selective reflection, and these properties are exploited in a number of optical applications.
Blue Phases
Blue phases are special types of liquid crystal phases that appear in the temperature range between a chiral nematic phase and an isotropic liquid phase. Blue phases have a regular three-dimensional cubic structure of defects with lattice periods of several hundred nanometers, and thus they exhibit selective Bragg reflections in the wavelength range of light (visible part of electromagnetic radiation) corresponding to the cubic lattice. Although blue phases are of interest for fast light modulators or tunable photonic crystals, the very narrow temperature range within which blue phases exist, usually less than a few kelvin, has always been a problem. Recently the stabilization of blue phases over a temperature range of more than 60 K including room temperature (260–326 K) could be demonstrated .
Furthermore, electro-optical switching with response times of the order of 10-4 s for the stabilized blue phases at room temperature has been shown .
In May 2008 it was announced that the first Blue Phase Mode LCD panel had been developed.
Discotic phases
Disk-shaped mesogens can orient themselves in a layer-like fashion known as the discotic nematic phase. If the disks pack into stacks, the phase is called a discotic columnar. The columns themselves may be organized into rectangular or hexagonal arrays. Chiral discotic phases, similar to the chiral nematic phase, are also known.
Lyotropic liquid crystals
A lyotropic liquid crystal consists of two or more components that exhibit liquid-crystalline properties in certain concentration ranges. In the lyotropic phases, solvent molecules fill the space around the compounds to provide fluidity to the system. In contrast to thermotropic liquid crystals, these lyotropics have another degree of freedom of concentration that enables them to induce a variety of different phases.
A compound which has two immiscible hydrophilic and hydrophobic parts within the same molecule is called an amphiphilic molecule. Many amphiphilic molecules show lyotropic liquid-crystalline phase sequences depending on the volume balances between the hydrophilic part and hydrophobic part. These structures are formed through the micro-phase segregation of two incompatible components on a nanometer scale. Soap is an everyday example of a lyotropic liquid crystal.
The content of water or other solvent molecules changes the self-assembled structures. At very low amphiphile concentration, the molecules will be dispersed randomly without any ordering. At slightly higher (but still low) concentration, amphiphilic molecules will spontaneously assemble into micelles or vesicles. This is done so as to 'hide' the hydrophobic tail of the amphiphile inside the micelle core, exposing a hydrophilic (water-soluble) surface to aqueous solution. These spherical objects do not order themselves in solution, however. At higher concentration, the assemblies will become ordered. A typical phase is a hexagonal columnar phase, where the amphiphiles form long cylinders (again with a hydrophilic surface) that arrange themselves into a roughly hexagonal lattice. This is called the middle soap phase. At still higher concentration, a lamellar phase (neat soap phase) may form, wherein extended sheets of amphiphiles are separated by thin layers of water. For some systems, a cubic (also called viscous isotropic) phase may exist between the hexagonal and lamellar phases, wherein spheres are formed that create a dense cubic lattice. These spheres may also be connected to one another, forming a bicontinuous cubic phase.
The objects created by amphiphiles are usually spherical (as in the case of micelles), but may also be disc-like (bicelles), rod-like, or biaxial (all three micelle axes are distinct). These anisotropic self-assembled nano-structures can then order themselves in much the same way as liquid crystals do, forming large-scale versions of all the thermotropic phases (such as a nematic phase of rod-shaped micelles).
For some systems, at high concentration, inverse phases are observed. That is, one may generate an inverse hexagonal columnar phase (columns of water encapsulated by amphiphiles) or an inverse micellar phase (a bulk liquid crystal sample with spherical water cavities).
A generic progression of phases, going from low to high amphiphile concentration, is:
- Discontinuous cubic phase ( micellar cubic phase)
- Hexagonal phase (hexagonal columnar phase) (middle phase)
- Bicontinuous cubic phase
- Lamellar phase
- Bicontinuous cubic phase
- Reverse hexagonal columnar phase
- Inverse cubic phase (Inverse micellar phase)
Even within the same phases, their self-assembled structures are tunable by the concentration: for example, in lamellar phases, the layer distances increase with the solvent volume. Since lyotropic liquid crystals rely on a subtle balance of intermolecular interactions, it is more difficult to analyze their structures and properties than those of thermotropic liquid crystals.
Similar phases and characteristics can be observed in immiscible diblock copolymers.
Metallotropic liquid crystals
Liquid crystal phases can also be based on low-melting inorganic phases like ZnCl2 that have a structure formed of linked tetrahedra and easily form glasses. The addition of long chain soaplike molecules leads to a series of new phases that show a variety of liquid crystalline behaviour both as a function of the inorganic-organic composition ratio and of temperature. This class of materials has been named metallotropic J.D. Martin et al.
Biological liquid crystals
Lyotropic liquid-crystalline phases are abundant in living systems, the study of which is referred to as polymorphism. Accordingly, lyotropic liquid crystals attract particular attention in the field of biomimetic chemistry. In particular, biological membranes and cell membranes are a form of liquid crystal. Their constituent molecules (e.g., phospholipids) are perpendicular to the membrane surface, yet the membrane is capable of a range of elastic stress, leading to some aspects of elastic behaviour to be exhibited. These lipids vary in shape (see page on lipid polymorphism). The constituent molecules can inter-mingle easily, but tend not to leave the membrane due to the high energy requirement of this process. Lipid molecules can flip from one side of the membrane to the other, this process being catalysed by flippases and floppases (depending on the direction of movement). These liquid crystal membrane phases can also host important proteins such as receptors freely "floating" inside, or partly outside, the membrane, e.g. CCT.
Many other biological structures exhibit LC behaviour. For instance, the concentrated protein solution that is extruded by a spider to generate silk is, in fact, a liquid crystal phase. The precise ordering of molecules in silk is critical to its renowned strength. DNA and many polypeptides can also form LC phases and this too forms an important part of current academic research.
Pattern formation in liquid crystals
Anisotropy of liquid crystals is a property not observed in other fluids. This anisotropy makes flows of liquid crystals to behave much differentially than ordinary fluids. For example, injecting a flux of a liquid crystal between two close parallel plates ( viscous fingering), orientation of molecules couples with the flow, with the result of emergence of dendritic patterns.. This anisotropy is also manifested in the interfacial energy (surface tension) between different liquid crystal phases. This anisotropy determines the equilibrium shape at the coexistence temperature, and is so strong that usually facets appear. When temperature is changed one of the phases grows, forming different morphologies depending on the temperature change. Since growth is controlled by heat diffusion, anisotropy in thermal conductivity favors growth in specific directions, which has also an effect on the final shape.
Theoretical treatment of liquid crystals
Microscopic theoretical treatment of fluid phases can become quite involved, owing to the high material density, which means that strong interactions, hard-core repulsions, and many-body correlations cannot be ignored. In the case of liquid crystals, anisotropy in all of these interactions further complicates analysis. There are a number of fairly simple theories, however, that can at least predict the general behaviour of the phase transitions in liquid crystal systems.
Order parameter
The description of liquid crystals involves an analysis of order. To make this quantitative, an orientational order parameter is usually defined based on the average of the second Legendre polynomial:
where 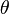 is the angle between the mesogen molecule axis and the local director (which is the 'preferred direction' in a volume element of a liquid crystal sample, also representing its local optical axis). This definition is convenient, since for a completely random and isotropic sample, S=0, whereas for a perfectly aligned sample S=1. For a typical liquid crystal sample, S is on the order of 0.3 to 0.8, and generally decreases as the temperature is raised. In particular, a sharp drop of the order parameter to 0 is observed when the system undergoes a phase transition from an LC phase into the isotropic phase. The order parameter can be measured experimentally in a number of ways. For instance, diamagnetism, birefringence, Raman scattering, NMR and EPR can also be used to determine S.
is the angle between the mesogen molecule axis and the local director (which is the 'preferred direction' in a volume element of a liquid crystal sample, also representing its local optical axis). This definition is convenient, since for a completely random and isotropic sample, S=0, whereas for a perfectly aligned sample S=1. For a typical liquid crystal sample, S is on the order of 0.3 to 0.8, and generally decreases as the temperature is raised. In particular, a sharp drop of the order parameter to 0 is observed when the system undergoes a phase transition from an LC phase into the isotropic phase. The order parameter can be measured experimentally in a number of ways. For instance, diamagnetism, birefringence, Raman scattering, NMR and EPR can also be used to determine S.
One could also characterize the order of a liquid crystal using other even Legendre polynomials (all the odd polynomials average to zero since the director can point in either of two antiparallel directions). These higher-order averages are more difficult to measure, but can yield additional information about molecular ordering.
Onsager hard-rod model
A very simple model which predicts lyotropic phase transitions is the hard-rod model proposed by Lars Onsager. This theory considers the volume excluded from the center-of-mass of one idealized cylinder as it approaches another. Specifically, if the cylinders are oriented parallel to one another, there is very little volume that is excluded from the center-of-mass of the approaching cylinder (it can come quite close to the other cylinder). If, however, the cylinders are at some angle to one another, then there is a large volume surrounding the cylinder where the approaching cylinder's centre-of-mass cannot enter (due to the hard-rod repulsion between the two idealized objects). Thus, this angular arrangement sees a decrease in the net positional entropy of the approaching cylinder (there are fewer states available to it).
The fundamental insight here is that, while parallel arrangements of anisotropic objects leads to a decrease in orientational entropy, there is an increase in positional entropy. Thus in some case greater positional order will be entropically favorable. This theory thus predicts that a solution of rod-shaped objects will undergo a phase transition, at sufficient concentration, into a nematic phase. Recently this theory is used to observe the phase transition between nematic and smectic-A at very high concentration also Hanif et al.. Although this model is conceptually helpful, its mathematical formulation makes several assumptions that limit its applicability to real systems.
Maier-Saupe mean field theory
This statistical theory includes contributions from an attractive intermolecular potential. The anisotropic attraction stabilizes parallel alignment of neighboring molecules, and the theory then considers a mean-field average of the interaction. Solved self-consistently, this theory predicts thermotropic phase transitions, consistent with experiment.
Elastic continuum theory
In this formalism, a liquid crystal material is treated as a continuum; molecular details are entirely ignored. Rather, this theory considers perturbations to a presumed oriented sample. One can identify three types of distortions that could occur in an oriented sample: (1) twists of the material, where neighboring molecules are forced to be angled with respect to one another, rather than aligned; (2) splay of the material, where bending occurs perpendicular to the director; and (3) bend of the material, where the distortion is parallel to the director and mesogen axis. All three of these types of distortions incur an energy penalty. They are defects that often occur near domain walls or boundaries of the enclosing container. The response of the material can then be decomposed into terms based on the elastic constants corresponding to the three types of distortions. Elastic continuum theory has been shown to be a particularly powerful tool for modelling liquid crystal devices.
Effect of chirality
As already described, chiral mesogens usually give rise to chiral mesophases. For molecular mesogens, this means that the molecule must possess some form of asymmetry, usually a stereogenic centre. An additional requirement is that the system not be racemic: a mixture of right- and left-handed versions of the mesogen will cancel the chiral effect. Due to the cooperative nature of liquid crystal ordering, however, a small amount of chiral dopant in an otherwise achiral mesophase is often enough to select out one domain handedness, making the system overall chiral.
Chiral phases usually have a helical twisting of the mesogens. If the pitch of this twist is on the order of the wavelength of visible light, then interesting optical interference effects can be observed. The chiral twisting that occurs in chiral LC phases also makes the system respond differently to right- and left-handed circularly polarized light. These materials can thus be used as polarization filters.
It is possible for chiral mesogens to produce essentially achiral mesophases. For instance, in certain ranges of concentration and molecular weight, DNA will form an achiral line hexatic phase. A curious recent observation is of the formation of chiral mesophases from achiral mesogens. Specifically, bent-core molecules (sometimes called banana liquid crystals) have been shown to form liquid crystal phases that are chiral. In any particular sample, various domains will have opposite handedness, but within any given domain, strong chiral ordering will be present. The appearance mechanism of this macroscopic chirality is not yet entirely clear. It appears that the molecules stack in layers and orient themselves in a tilted fashion inside the layers. These liquid crystals phases may be ferroelectric or anti- ferroelectric, both of which are of interest for applications.
Chirality can also be incorporated into a phase by adding a chiral dopant, which needs not necessarily be mesogenic itself. TN or STN mixtures often contain a small amount of such dopants.
Applications of liquid crystals
Liquid crystals find wide use in liquid crystal displays, which rely on the optical properties of certain liquid crystalline substances in the presence or absence of an electric field. In a typical device, a liquid crystal layer (typically 10 μm thick) sits between two polarizers that are crossed (oriented at 90° to one another). The liquid crystal alignment is chosen so that its relaxed phase is a twisted one (see Twisted nematic field effect). This twisted phase reorients light that has passed through the first polarizer, allowing it to be transmitted through the second polarizer (and reflected back to the observer if a reflector is provided). The device thus appears transparent. When an electric field is applied to the LC layer, the long molecular axes tend to align parallel to the electric field thus gradually untwisting in the center of the liquid crystal layer. In this state, the mesogens do not reorient light, so the light polarized at the first polarizer is absorbed at the second polarizer, and the device loses transparency with increasing voltage. In this way, the electric field can be used to make a pixel switch between transparent or opaque on command. Color LCD systems use the same technique, with colour filters used to generate red, green, and blue pixels. Similar principles can be used to make other liquid crystal based optical devices.
Thermotropic chiral LCs whose pitch varies strongly with temperature can be used as crude thermometers, since the color of the material will change as the pitch is changed. Liquid crystal color transitions are used on many aquarium and pool thermometers. Other liquid crystal materials change colour when stretched or stressed. Thus, liquid crystal sheets are often used in industry to look for hot spots, map heat flow, measure stress distribution patterns, and so on. Liquid crystal in fluid form is used to detect electrically generated hot spots for failure analysis in the semiconductor industry. Liquid crystal memory units with extensive capacity were used in Space Shuttle navigation equipment.
It is also worth noting that many common fluids are in fact liquid crystals. Soap, for instance, is a liquid crystal, and forms a variety of LC phases depending on its concentration in water.