Tsetse fly
Did you know...
SOS Children, which runs nearly 200 sos schools in the developing world, organised this selection. Sponsor a child to make a real difference.
| Tsetse fly (Glossina) | |
|---|---|
 |
|
| Conservation status | |
|
Secure
|
|
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Diptera |
| Suborder: | Brachycera |
| Subsection: | Calyptratae |
| Superfamily: | Hippoboscoidea |
| Family: | Glossinidae Theobald, 1903 |
| Genus: | Glossina Wiedemann, 1830 |
| Species groups | |
|
|
Tsetse (pronounced /ts/e-/ts/e, teet-SEE, or set-see) are large biting flies from Africa which live by feeding on the blood of vertebrate animals. Tsetse include all the species in the genus Glossina, which are generally placed in their own family, Glossinidae.
Tsetse have been extensively studied because they are biological vectors of the African trypanosomiases, deadly diseases which include sleeping sickness in people and nagana in cattle.
Tsetse are crudely similar to other large flies, such as the housefly, but can be distinguished by four characteristics of their anatomy, two of which are easy to observe. Tsetse fold their wings completely when they are resting so that one wing rests directly on top of the other over their abdomen. Tsetse also have a long proboscis which extends directly forward and is attached by a distinct bulb to the bottom of their head.
Tsetse have existed in the modern morphological form for at least 34 million years since fossil tsetse have been recovered from the Florissant Fossil Beds in Colorado.
Biology
The biology of tsetse is relatively well understood. Tsetse have been extensively studied because of their medical, veterinary, and economic importance, because the flies can be raised in a laboratory, and because the flies are relatively large, facilitating their analysis. Entomologists have discovered a great deal about tsetse morphology, anatomy, development, and metabolism.
Morphology
Tsetse can be seen as independent individuals in two forms: as third instar larvae, as adults.
Tsetse first become separate from their mothers during the third larval instar, during which they have the typical appearance of maggots. However, this life stage is short, lasting at most a few hours, and is almost never observed outside of the laboratory.
Tsetse next become puparia—small, hard shelled, oblongs with two distinctive, small, dark lobes at one end. Tsetse puparia are under 1.0 cm long. Within the puparial shell, tsetse complete the last two larval instars and the pupal stage.
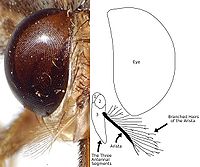
Tsetse then emerge as adult flies. Tsetse adults are relatively large flies, with lengths of ½–1½ cm , and have a recognizable shape or bauplan so they can usually be distinguished without trouble from other flies. Tsetse have large heads, distinctly separated eyes, and unusual antennae. The tsetse thorax is quite large, while the abdomen is wide rather than elongated and shorter than the wings.
Four characteristics definitively separate adult tsetse from other kinds of flies:
| Proboscis | Tsetse have a distinct proboscis, a long thin structure attached to the bottom of the head and pointing forward. | |
| Folded wings | When at rest, tsetse fold their wings completely one on top of the other. | |
| Hatchet cell | The discal medial ("middle") cell of the wing has a characteristic hatchet shape resembling a meat cleaver or a hatchet. | |
| Branched arista hairs | The antennae have arista with hairs which are themselves branched. |
Anatomy
Like all other insects, Tsetse flies have an adult body comprising three visibly distinct parts: the head, the thorax and the abdomen.
The head has large eyes, distinctly separated on each side, and a distinct, forward-pointing proboscis attached underneath by a large bulb. The thorax is large, made of three fused segments. Three pairs of legs are attached to the thorax, as are two wings and two halteres. The abdomen is short but wide and changes dramatically in volume during feeding.
The internal anatomy of tsetse is fairly typical of the insects. The crop is large enough to accommodate a huge increase in size during the bloodmeal since tsetse can take a bloodmeal weighing as much as themselves. The reproductive tract of adult females includes a uterus which can become large enough to hold the third instar larva at the end of each pregnancy.
Most tsetse flies are physically very tough indeed. Houseflies are easily killed with a fly-swatter but it takes a great deal of effort to crush a tsetse fly.
Life cycle
Tsetse have an unusual life cycle which may be due to the richness of their food source. Female tsetse only fertilize one egg at a time and retain each egg within their uterus to have the offspring develop internally during the first larval stages, a strategy called adenotrophic viviparity. During this time, the female feeds the developing offspring with a milky substance which is secreted by a modified gland in the uterus. In the third larval stage, the tsetse larva finally leave the uterus and begin their independent life. However, the newly independent tsetse larva simply crawls into the ground, forms a hard outer shell called the puparial case in which it completes its morphological transformation into an adult fly. This lifestage has a variable duration, generally twenty to thirty days, and the larva must rely on stored resources during this time. The importance of the richness of blood to this development can be seen since all tsetse development prior to the emergence from the puparial case as a full adult occurs without feeding based only on nutritional resources provided by the female parent. The female must obtain enough energy for her needs, for the needs of her developing offspring, and to store the resources which her offspring will require until it emerges as an adult.
Technically these insects undergo the standard development process of insects which comprises oocyte formation, ovulation and fertilization, development of the egg, five larval stages, a pupal stage, and the emergence and maturation of the adult.
General biology
Tsetse has three distinct symbionts. The primary symbiont is Wigglesworthia within bacteriocyte, the secondary symbiont is Sodalis intercellularly or intracellularly, and the third is some kind of Wolbachia.
The tsetse Glossina palpalis is also a vector and host of Hepatozoon petti, a parasitic Sporozoa of the nile crocodile.
Systematics
Tsetse include up to thirty four species and sub-species depending on the particular classification used.
Tsetse are sufficiently different in appearance and behaviour to have been placed in their own distinct branch of the flies. This placement is controversial. The science of systematics is currently struggling to reconcile the traditional form of biological classification with the modern understanding of genomic evolution and speciation. The controversy surrounding the placement of tsetse is therefore likely to continue into the future.
All current classifications place all the tsetse species in a single genus named Glossina. Most classifications place this genus as the sole member of the family Glossinidae. The Glossinidae are generally placed within the superfamily Hippoboscoidea, which contains other hematophagous families. This superfamily is in the subsection Calyptratae which includes the housefly and the blowfly due to the similarity of their developmental biology. This infraorder in turn, is part of the sub-order Brachycera, the stubby flies with reduced antenna.
Species
The tsetse genus is generally split into three different groups of species based on a combination of distributional, behavioural, and morphological characteristics. The genus includes :
|
|
|
Trypanosomiasis
Tsetse are biological vectors of trypanosomes meaning that tsetse, in the process of feeding, acquire and then transmit small, single-celled organisms called trypanosomes from infected vertebrate hosts to uninfected animals. Some tsetse transmitted trypanosome species cause trypanosomiasis, an infectious disease. In humans, tsetse transmitted trypanosomiasis is called sleeping sickness. In animals, tsetse vectored trypanosomiases include nagana, souma, and surra according to the animal infected and the trypanosome species involved, although the usage is not strict and nagana is occasionally used for any form of animal trypanosomiasis.
Trypanosomes are animal parasites, specifically protozoa of the genus Trypanosoma. These organisms are approximately the size of red blood cells. Different species of trypanosomes infect different hosts as can be seen in the table attached to this section. Trypanosomes range widely in their effects on the vertebrate hosts. Some species, such as Trypanosoma theileri, do not seem to cause any health problems except perhaps in animals which are already quite sick .
Some strains are much more virulent. Tsetse seem to be unaffected by the infection of trypanosomes but it is entirely possible that the parasites alter tsetse behaviour or have other effects which improve the chances of transmission and survival. These trypanosomes have become highly evolved and developed a life cycle which requires periods in both the vertebrate and tsetse hosts.
Tsetse transmit trypanosomes in two ways, mechanical and biological transmission.
- Mechanical transmission involves the direct transmission of the same individual trypanosomes taken from an infected host into an uninfected host. The name mechanical reflects the similarity of this mode of transmission to the transmission which could be caused mechanically with a syringe. Mechanical transmission requires that tsetse feed on an infected host and acquire trypanosomes in the bloodmeal, and then, within in a relatively short period, for tsetse to feed on an uninfected host and regurgitate some of the infected blood from the first bloodmeal into the tissue of the uninfected animal. This type of transmission occurs most frequently when tsetse are interrupted during a bloodmeal and attempt to satiate themselves with another meal. Other flies, such as horse-flies, also can cause mechanical transmission of trypanosomes .
- Biological transmission requires a period of incubation of the trypanosomes within the tsetse host. The term biological is used because trypanosomes must reproduce through several generations inside the tsetse host during the period of incubation, which requires extreme adaptation of the trypanosomes to their tsetse host. In this mode of transmission, trypanosomes reproduce through several generations, changing in morphology at certain periods. This mode of transmission also includes the sexual phase of the trypanosomes. Tsetse are believed to be more likely to become infected by trypanosomes during their first few bloodmeals. Tsetse infected by trypanosomes are thought to remain infected for the remainder of their lives. Because of the adaptations required for biological transmission, trypanosomes which are transmitted biologically by tsetse cannot be transmitted in this manner by other insects.
The relative importance of these two modes of transmission for the propagation of tsetse-vectored trypanosomiases is not yet well understood. However, since the sexual phase of the trypanosome lifecycle occurs within the tsetse host, biological transmission is a required step in the life cycle of the tsetse vectored trypanosomes.
The cycle of biological transmission of trypanosomiasis involves two phases, one inside the tsetse host and the other inside the vertebrate host. Trypanosomes are not passed between a pregnant tsetse and her offspring so all newly emerged tsetse adults are free of infection. An uninfected fly which feeds upon an infected vertebrate animal may acquire trypanosomes in its proboscis or gut. These trypanosomes, depending on the species, may remain in place, move to a different part of the digestive tract, or migrate through the tsetse body into the salivary glands. When an infected tsetse bites a susceptible host, the fly may regurgitate part of a previous bloodmeal which contains trypanosomes or may inject trypanosomes contained within its saliva. It is believed that the inoculation must contain a minimum of 300 to 450 individual trypanosomes to be successful, and may contain up to 40,000 individuals .
The trypanosomes are injected into vertebrate muscle tissue but make their way, first into the lymphatic system, then into the bloodstream, and eventually into the brain. The disease causes the swelling of the lymph glands, emaciation of the body, and eventually leads to death. Uninfected tsetse may bite the infected animal prior to its death and acquire the disease, thereby closing the transmission cycle.
The tsetse vectored trypanosomiases affect various vertebrate species including humans, antelopes, bovine cattle, camels, horses, sheep, goats, and pigs. These diseases are caused by several different trypanosome species which may also survive in wild animals such as crocodiles and monitor lizards. The diseases have different distributions across the African continent and are therefore transmitted by different species of tsetse. The following table summarizes this information :
| Disease | Species affected | Trypanosoma agents | Distribution | Glossina vectors |
|---|---|---|---|---|
| Sleeping sickness — chronic form | humans | T. brucei gambiense | Western Africa | G. palpalis G. tachinoides G. fuscipes G. morsitans |
| Sleeping sickness — acute form | humans | T. brucei rhodesiense | Eastern Africa | G. morsitans G. swynnertoni G. pallidipes G. fuscipes |
| Nagana — acute form | antelope cattle camels horses |
T. brucei brucei | Africa | G. morsitans G. swynnertoni G. pallidipes G. palpalis G. tachinoides G. fuscipes |
| Nagana — chronic form | cattle camels horses |
T. congolense | Africa | G. palpalis G. morsitans G. austeni G. swynnertoni G. pallidipes G. longipalpis G. tachinoides G. brevipalpis |
| Nagana — acute form | domestic pigs cattle camels horses |
T. simiae | Africa | G. palpalis G. fuscipes G. morsitans G. tachinoides G. longipalpis G. fusca G. tabaniformis G. brevipalpis G. vanhoofi G. austeni |
| Nagana — acute form | cattle camels horses |
T. vivax | Africa | G. morsitans G. palpalis G. tachinoides G. swynnertoni G. pallidipes G. austeni G. vanhoofi G. longipalpis |
| Surra — chronic form | domestic pigs warthog (Phacochoerus aethiopicus) forest hogs ( Hylochoerus spp.) |
T. suis | Africa | G. palpalis G. fuscipes G. morsitans G. tachinoides G. longipalpis G. fusca G. tabaniformis G. brevipalpis G. vanhoofi G. austeni |
Human trypanosomiasis
Human African trypanosomiasis, also called sleeping sickness, is caused by trypanosomes of the Trypanosoma brucei species. This disease is invariably fatal unless treated but can almost always be cured with current medicines, if the disease is diagnosed early enough.
Sleeping sickness begins with a tsetse bite leading to an inoculation in the sub-cutaneous tissue. The infection moves into the lymphatic system leading to a characteristic swelling of the lymph glands which is called Winterbottoms's sign . The infection progresses into the blood stream and eventually crosses into the central nervous system and invades the brain leading to extreme lethargy and eventually to death.
The Trypanosoma brucei species, which causes the disease, has often been subdivided into three sub-genera which were identified based either on the vertebrate hosts which the strain could infect or on the virulence of the disease in humans. The trypanosomes infectious to animals and not to humans were named Trypanosoma brucei brucei. The strains which infected humans were divided into two sub-species based on their different virulences: Trypanosoma brucei gambiense was thought to have a slower onset and Trypanosoma brucei rhodesiense refers to strains with a more rapid, virulent onset. This characterization has always been problematic but was the best that could be done given the knowledge of the time and the tools available for identification. A recent molecular study using restriction fragment length polymorphism analysis suggests that the three sub-genera are polyphyletic , so the elucidation of the strains of T. brucei infective to humans will require a more complex explanation.
Other forms of human trypanosomiasis also exist but are not transmitted by tsetse. The most notable is American trypanosomiasis, known as Chagas disease, which occurs in South America, caused by Trypanosoma cruzi, and transmitted by certain species of the Reduviidae, members of the Hemiptera.
Animal trypanosomiasis
Animal trypanosomiasis, also called nagana when it occurs in bovine cattle or horses or sura when it occurs in domestic pigs, is caused by several trypanosome species. These diseases reduce the growth rate, milk productivity, and strength of farm animals, generally leading to the eventually death of the infected animals. Certain species of cattle are called trypanotolerant because they can survive and grow even when infected with trypanosomes although they also have lower productivity rates when infected.
The course of the disease in animals is similar to the course of sleeping sickness in humans.
Trypanosoma congolense and Trypanosoma vivax are the two most important species infecting bovine cattle in sub-saharan Africa. Trypanosoma simiae causes a virulent disease in swine.
Other forms of animal trypanosomiasis are also known from other areas of the globe, caused by different species of trypanosomes and transmitted without the intervention of the tsetse fly.
Tsetse vector ranges mostly in the central part of Africa.
Control
Tsetse control has been undertaken in order to reduce the incidence of the diseases which the flies transmit. Two alternative strategies have been used in the attempts to reduce the African trypanosomiases. One tactic is primarily medical or veterinary and targets the disease directly using monitoring, prophylaxis, treatment, and surveillance to reduce the number of organisms which carry the disease. The second strategy is generally entomological and intends to disrupt the cycle of transmission by reducing the number of flies.
The idea of tsetse control implies a change in the relationship between people and these insects. Prior to the twentieth century, people in Africa had largely adapted to the presence of tsetse. Human settlement patterns and agricultural practices had adapted to the presence of the fly. For example, in Ethiopia draft powered farming was restricted to the highland areas where the flies were absent whereas lowland areas where tsetse are present were more sparsely populated by people living a nomadic, less agriculturally intensive lifestyle. Tsetse control is a response to changing conditions. Tsetse control has been proposed as a way of reducing the incidence of the disease in the populations living in tsetse regions, of allowing the expansion of human settlement and agriculture into new areas, and of helping people previously relocated either in forced transfers or due to migration.
Tsetse control efforts have been undertaken throughout the African continent but long-term, sustainable control has rarely been achieved. Tsetse control efforts invariably are tied to the complex problems of poverty, heath, politics, and violence which have proved such a disaster for the African people.
The reduction of fly numbers has generally been attempted with two different aims, either eradication which intends to completely eliminate tsetse from the area or control which aims simply to reduce the numbers. Eradication is an idea which has often been imagined, has repeatedly been attempted, and is still proposed but many reasons suggest that control is a safer, cheaper, more realistic, and sustainable approach. Eradication refers to the successful killing of every tsetse either in a region or, under more grandiose proposals, from the entire African continent. Local eradication efforts have repeatedly been undertaken and have achieved temporary success only to fail in the long term because tsetse were able to re-invade (Zanzibar).
All of the economic, ecological, political, and environmental justifications for eradication have been called into question. The economic justification for eradication offsets the immense costs of the eradication campaign against the medical and veterinary benefits which are considered to accrue in perpetuity.
However, eradication campaigns may have unintended social consequences, as a successful campaign may open up lands for agriculture which have previously been populated by nomadic hunters, resulting in the displacement of the original population with its attendant consequences.
Control techniques
Many techniques have been used to reduce tsetse populations with earlier crude methods being replaced in more recent times by methods which are cheaper, more directed, and ecologically better considered.
Slaughter of wild animals
One early technique involved the slaughter of all the wild animals on which tsetse fed. For example, the island of Principé off the west coast of Africa, was entirely cleared of feral pigs in the 1930s which led to the extirpation of the fly. While the fly eventually re-invaded in the 1950s, the new population of tsetse was free from the disease.
Land clearing
Another early technique involved the complete removal of any brush or woody vegetation from an area. Tsetse tend to rest on the trunks of trees so the removal of woody vegetation made the area inhospitable to the flies. However, the technique has not been widely used and has been abandoned in more recent times. Preventing the regrowth of woody vegetation requires continuous clearing efforts which is only practicable where large human populations are present. The clearing of woody vegetation has come to be seen as an environmental problem more than a benefit.
Pesticide campaigns
Pesticides have been used to control tsetse starting initially during the early part of the twentieth century in localized efforts using the inorganic metal based pesticides, expanding after the Second World war into massive aerial and ground based campaigns using the early organic pesticides such as DDT, and continuing with the targeted use of pour-ons in which advanced organic pesticides are applied directly to the backs of cattle.
Trapping
Tsetse populations can be monitored and effectively controlled using simple, inexpensive traps. These often use electric blue cloth, since this colour attracts the flies. Early traps mimicked the form of cattle but this seems unnecessary and recent traps are simple sheets or have a biconical form. The traps can kill by channeling the flies into a collection chamber or by exposing the flies to insecticide sprayed on the cloth.
The use of chemicals as attractants to lure tsetse to the traps has been studied extensively in the late 20th century, but this has mostly been of interest to scientists rather than as an economically reasonable solution. The attractants studied have been those which might be used by tsetse to find their food, like carbon dioxide and acetone, which are given off in the animals' breath and distributed downwind to form an 'odour plume'. Synthetic versions of these chemicals can be used to create artificial odour plumes. A cheaper approach is to place some cattle urine in a half gourd near the trap. For large trapping efforts, the use of additional traps is generally cheaper than the use of expensive artificial attractants.
A special trapping method is applied in Ethiopia, where the BioFarm Consortium (ICIPE, BioVision Foundation, BEA, Helvetas, DLCO-EA, Praxis Ethiopia) applies the traps in a sustainable agriculture and rural development context (SARD). The traps are just the entry point, followed by improved farming, human health and marketing inputs. This method is in the final stage of testing (as per 2006).
Releases of irradiated males
The sterile insect technique has been used to reduce tsetse populations. This technique involves the rearing of large numbers of tsetse, separation of the males, irradiation of these flies with large doses of gamma rays to make them sterile and then release into to the wild. Since females only mate a few times in their life, generally only once, any mating with a sterile male will prevent that female from giving birth to any offspring.
The Sterile Insect Technique has recently been used on Zanzibar, an island off the coast of East Africa. Like other eradication efforts, early indications are that the fly numbers have been decimated with the fly possibly extirpated (locally eradicated) from the island. A number of traps are in place to monitor the island and repress any resurgence.
Additionally, using the parasite refractory strains is another method to control the tsetse, that means providing the blood meal containing the trypanocide before releasing the sterilised males. Also we can consider to use the cytoplasmic incompatibility (CI) strategy to control the population of tsetse. With the development of genetic engineering, the releasing of engineered parasite refractory counterparts is another strategy to control the population of tsetse.
Etymology
The word 'tsetse' comes from Tswana, a language of southern Africa, and, in that language, the word means fly. Recently 'tsetse' without the 'fly' has become more common in English, particularly in the scientific and development communities.
The pronunuciation of the word differs in different regions. Many African languages have an ejective ts sound and so a common pronunciation of the word involves two identical syllables both having this ts sound and a shorter sound of the vowel, as ts-eh-ts-eh. The British pronunciation of the word uses two different sounds for the two different syllables, generally tee-tsee. In Zimbabwe, it is generally pronounced tseh-tsee.