Fire
Did you know...
This Schools selection was originally chosen by SOS Children for schools in the developing world without internet access. It is available as a intranet download. A quick link for child sponsorship is http://www.sponsor-a-child.org.uk/
Fire is the heat and light energy released during a chemical reaction, in particular a combustion reaction. Depending on the substances alight, and any impurities within, the colour of the flame and the fire's intensity might vary.
Chemistry
Flaming fires
Flaming fires involve the chemical oxidation of a fuel ( combustion or release of energy) with associated flame, heat, and light. The flame itself occurs within a region of gas where intense exothermic reactions are taking place. An exothermic reaction is a chemical reaction whereby heat and energy are released as a substance changes to a more stable chemical form (in the case of fire, usually generating carbon dioxide and water). As chemical reactions occur within the fuel being burned, light and heat are released. Depending upon the specific chemical and physical change taking place within the fuel, the flame may or may not emit light in the visible spectrum. For example, burning alcohol or burning hydrogen is usually invisible to the naked eye although the heat given off is tremendous.
The visible flame has little mass, and it is comprised of luminous gases which emit energy (photons) as part of the oxidation process. The colour of the flame is dependent upon the energy level of the photons emitted. Lower energy levels produce colors toward the red end of the light spectrum while higher energy levels produce colors toward the blue end of the spectrum. The hottest flames are white in appearance. The colour of a fire may also be affected by chemical elements in the flame, such as barium giving a green flame color. The flame colour depends also on the unoxidized carbon particles. In some cases there is a partial fuel oxidation due to oxygen lack in the central part of the flame, where combustion reactions take place. In such cases the unoxidized hot carbon particles emit radiation in the light spectrum, resulting in a yellow/red flame, such that of a common house fireplace.
Chemical reaction
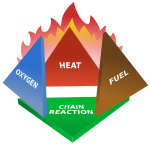
Fires start when a flammable and/or a combustible material with an adequate supply of oxygen or another oxidizer is subjected to enough heat and is able to sustain a chain reaction. This is commonly called the fire tetrahedron. No fire can exist without all of these elements being in place.
Once ignited, a chain reaction must take place whereby fires can sustain their own heat by the further release of heat energy in the process of combustion and may propagate, provided there is a continuous supply of an oxidizer and fuel.
Fire can be extinguished by removing any one of the elements of the fire tetrahedron. Fire extinguishing by the application of water acts by removing heat from the fuel faster than combustion generates it. Application of carbon dioxide is intended primarily to starve the fire of oxygen. A forest fire may be fought by starting smaller fires in advance of the main blaze, to deprive it of fuel. Other gaseous fire suppression agents, such as halon or HFC-227, interfere with the chemical reaction itself.
Flame
A flame is an exothermic, self-sustaining, oxidizing chemical reaction producing energy and glowing hot matter, of which a very small portion is plasma. It consists of reacting gases and solids emitting visible and infrared light, the frequency spectrum of which depends on the chemical composition of the burning elements and intermediate reaction products.
In many cases, such as the burning of organic matter, for example wood, or the incomplete combustion of gas, incandescent solid particles called soot produce the familiar red-orange glow of 'fire'. This light has a continuous spectrum. Complete combustion of gas has a dim blue colour due to the emission of single-wavelength radiation from various electron transitions in the excited molecules formed in the flame. Usually oxygen is involved, but hydrogen burning in chlorine also produces a flame, producing hydrogen chloride (HCl). Other possible combinations producing flames, amongst many more, are fluorine and hydrogen, and hydrazine and nitrogen tetroxide.
The glow of a flame is complex. Black-body radiation is emitted from soot, gas, and fuel particles, though the soot particles are too small to behave like perfect blackbodies. There is also photon emission by de-excited atoms and molecules in the gases. Much of the radiation is emitted in the visible and infrared bands. The colour depends on temperature for the black-body radiation, and on chemical makeup for the emission spectra. The dominant color in a flame changes with temperature. The photo of the forest fire is an excellent example of this variation. Near the ground, where most burning is occurring, the fire is white, the hottest color possible for organic material in general, or yellow. Above the yellow region, the colour changes to orange, which is cooler, then red, which is cooler still. Above the red region, combustion no longer occurs, and the uncombusted carbon particles are visible as black smoke.
The National Aeronautics and Space Administration (NASA) of the United States has recently found that gravity plays a role. Modifying the gravity causes different flame types. The common distribution of a flame under normal gravity conditions depends on convection, as soot tends to rise to the top of a general flame, as in a candle in normal gravity conditions, making it yellow. In microgravity or zero gravity, such as an environment in outer space, convection no longer occurs, and the flame becomes spherical, with a tendency to become more blue and more efficient (although it will go out if not moved steadily, as the CO2 from combustion does not disperse in microgravity, and tends to smother the flame). There are several possible explanations for this difference, of which the most likely is that the temperature is evenly distributed enough that soot is not formed and complete combustion occurs. Experiments by NASA reveal that diffusion flames in microgravity allow more soot to be completely oxidized after they are produced than diffusion flames on Earth, because of a series of mechanisms that behave differently in microgravity when compared to normal gravity conditions. These discoveries have potential applications in applied science and industry, especially concerning fuel efficiency.
In combustion engines, various steps are taken to eliminate a flame. The method depends mainly on whether the fuel is oil, wood, or a high-energy fuel such as jet fuel.
Typical temperatures of fires and flames
- Oxyhydrogen flame: 2000 °C or above (3645 °F)
- Bunsen burner flame: 1300 to 1600 °C (2372 to 2912 °F)
- Blowtorch flame: 1,300 °C (2372 °F)
- Candle flame: 1000 °C (1832 °F)
- Smoldering cigarette:
- Temperature without drawing: side of the lit portion; 400 °C (750 °F); middle of the lit portion: 585 °C (1110 °F)
- Temperature during drawing: middle of the lit portion: 700 °C (1290 °F)
- Always hotter in the middle.
Temperatures of flames by appearance
The temperature of flames with carbon particles emitting light can be assessed by their colour:
- Red
- Just visible: 525 °C (977 °F)
- Dull: 700 °C (1290 °F)
- Cherry, dull: 800 °C (1470 °F)
- Cherry, full: 900 °C (1650 °F)
- Cherry, clear: 1000 °C (1830 °F)
- Orange
- Deep: 1100 °C (2010 °F)
- Clear: 1200 °C (2190 °F)
- White
- Whitish: 1300 °C (2370 °F)
- Bright: 1400 °C (2550 °F)
- Dazzling: 1500 °C (2730 °F)
Controlling Fire
The ability to control fire was a major change in the habits of early humans. Making fire to generate heat and light made it possible for people to cook food, increasing the variety and availability of nutrients. Fire also kept nocturnal predators at bay. Archaeology indicates that ancestors or relatives of modern humans might have controlled fire as early as 790,000 years ago. The Cradle of Humankind site has evidence for controlled fire from 1 to 1.8 million years ago.
By the Neolithic Revolution, during the introduction of grain based agriculture, people all over the world used fire as a tool in landscape management. These fires were typically controlled burns or "cool fires", as opposed to uncontrolled "hot fires" that damage the soil. Hot fires destroy plants and animals, and endanger communities. This is especially a problem in the forests of today where traditional burning is prevented in order to encourage the growth of timber crops. Cool fires are generally conducted in the spring and fall. They clear undergrowth, burning up biomass that could trigger a hot fire should it get too dense. They provide a greater variety of environments, which encourages game and plant diversity. For humans, they make dense, impassable forests traversable.
The first technical application of the fire may have been the extracting and treating of metals. There are numerous modern applications of fire. In its broadest sense, fire is used by nearly every human being on earth in a controlled setting every day. Users of internal combustion vehicles employ fire every time they drive. Thermal power stations provide electricity for a large percentage of humanity.
The use of fire in warfare has a long history. Hunter-gatherer groups around the world have been noted as using grass and forest fires to injure their enemies and destroy their ability to find food, so it can be assumed that fire has been used in warfare for as long as humans have had the knowledge to control it. Homer detailed the use of fire by Greek commandos who hid in a wooden horse to burn Troy during the Trojan war. Later the Byzantine fleet used Greek fire to attack ships and men. In the First World War, the first modern flamethrowers were used by infantry, and were successfully mounted on armoured vehicles in the Second World War. In the latter war, incendiary bombs were used by Axis and Allies alike, notably on Rotterdam, London, Hamburg and, notoriously, at Dresden, in the latter two cases firestorms were deliberately caused in which a ring of fire surrounding each city was drawn inward by an updraft caused by a central cluster of fires. The United States Army Air Force also extensively used incendiaries against Japanese targets in the latter months of the war, devastating entire cities constructed primarily of wood and paper houses. In the Second World War, the use of napalm and molotov cocktails was popularized, though the former did not gain public attention until the Vietnam War. More recently many villages were burned during the Rwandan Genocide.
Fire and fuel

Setting fuel aflame releases usable energy. Wood was a prehistoric fuel, and is still viable today. The use of fossil fuels, such as petroleum, natural gas and coal, in power plants supplies the vast majority of the world's electricity today; the International Energy Agency states that nearly 80% of the world's power comes from these sources. The fire in a power station is used to heat water, creating steam that drives turbines. The turbines then spin an electric generator to produce power.
The unburnable solid remains of a combustible material left after a fire is called clinker if its melting point is below the flame temperature, so that it fuses and then solidifies as it cools, and ash if its melting point is above the flame temperature. Incomplete combustion of a carbonaceous fuel can result in the production of soot.
Fire protection and prevention
Fire fighting services are provided in most developed areas to extinguish or contain uncontrolled fires. Trained firefighters use fire trucks, water supply resources such as water mains and fire hydrants or they might use A and B class foam depending on what is feeding the fire. An array of other equipment to combat the spread of fires.
Fire prevention is intended to reduce sources of ignition, and is partially focused on programs to educate people from starting fires. Buildings, especially schools and tall buildings, often conduct fire drills to inform and prepare citizens on how to react to a building fire. Purposely starting destructive fires constitutes arson and is a criminal offense in most jurisdictions.
Model building codes require passive fire protection and active fire protection systems to minimize damage resulting from a fire. The most common form of active fire protection is fire sprinklers. To maximize passive fire protection of buildings, building materials and furnishings in most developed countries are tested for fire-resistance, combustibility and flammability. Upholstery, carpeting and plastics used in vehicles and vessels are also tested.
Fire classifications
In order to facilitate consistent extinguishment approaches, and maximize occupant and fire fighter safety, fires are classified using code letters in many countries. Below is a table showing the standard operated in Europe and Australasia against the system used in the United States.
| Type of Fire | European/Australian Classification | United States Classification |
|---|---|---|
| Fires that involve flammable solids such as wood, cloth, rubber, paper, and some types of plastics. | Class A | Class A |
| Fires that involve flammable liquids or liquifiable solids such as petrol/gasoline, oil, paint, some waxes & plastics, but not cooking fats or oils | Class B | Class B |
| Fires that involve flammable gases, such as natural gas, hydrogen, propane, butane | Class C | |
| Fires that involve combustible metals, such as sodium, magnesium, and potassium | Class D | Class D |
| Fires that involve any of the materials found in Class A and B fires, but with the introduction of an electrical appliances, wiring, or other electrically energized objects in the vicinity of the fire, with a resultant electrical shock risk if a conductive agent is used to control the fire | Class E | Class C |
| Fires involving cooking fats and oils. The high temperature of the oils when on fire far exceeds that of other flammable liquids making normal extinguishing agents ineffective. | Class F | Class K |
Burns
Fire causes injury in forms of first-, second-, and third-degree burns. A first-degree burn damages the epidermis only, while a second-degree burn goes through the epidermis and dermis. A third-degree burn destroys both the epidermis and dermis, and kills all nerve receptors underneath the skin. A common result of second- and third-degree burns is large amounts of granulation tissue, or scar tissue, in place of the burnt skin.
Practical uses

Fire is or has been used:
- For light, heat (for cooking, survival and comfort), and protection
- As a weapon of warfare, especially during ancient and medieval times, but also used in modern day warfare
- For fire-stick farming
- For cremation
- For welding
- For celebration (such as, birthday candles)
- For back-burning in fighting fires
- For controlled burn-offs for preventing wildfires
- For controlled burn-offs to clear land for agriculture